

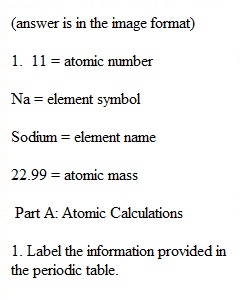
Q Atoms and Elements Assignment Name _________________________ Date__________________________ Directions: complete the assignment by answering each question. Please submit the assignment as an attachment file. Part A: Atomic Calculations 1. Label the information provided in the periodic table. 2. Complete the following table for neutral atoms. Nuclide Symbol Atomic Number Mass # Protons Neutrons Electrons 12 12 20 22 55 29 35 45 35 17 12050Sn 28 31 39 19 74 110 11 Na Sodium 22.99 3. An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu and a relative abundance of 42.6%. Find the atomic mass of this element and, referring to the periodic table, identify it. Show all mathematical calculations. 4. Complete the table. Chemical Symbol Group # Group Name Metal/Nonmetal Cl 7A(17) Ca Metal Xe Nonmetal Na Alkali Metal F Part B: Electron Configuration/Ions (1) Write the electron configuration for each of the following: (a) Magnesium________________________ (b) Chlorine__________________________ (c) Potassium ________________________ (d) Fluorine _________________________ (2) Determine the number of protons and electrons in each ion and write the electron dot symbol. (a) Al3+____________________________ (b) S2-_____________________________ (c) I-______________________________ (d) Ag+____________________________ (3) Predict how many electrons each element will most likely gain or lose. (a) I___________________________ (b) Ba__________________________ (c) Cs___________________________ (d) Se___________________________
View Related Questions